You may label your weighing bottle with your initials and its contents. The moles of calcium carbonate CaC0 3 are equal to the moles of.

Solved Experiment 1 Gravimetric Analysis With Calcium Chegg Com
Using gravimetric analysis it has been determined that the unknown Group 1 metal carbonate compound is K2CO3 potassium carbonate.
. By mixing a solution of the calcium halide with excess aqueous sodium carbonate calcium carbonate can be precipitated collected and weighed to determine the identity of the halogen in the. Sodium bicarbonate NaHCO3 Sodium bicarbonate is a weak short-acting antacid. By filtering and weighing the carbonate after it has precipitated the mass and moles of CaCO3 could then be found.
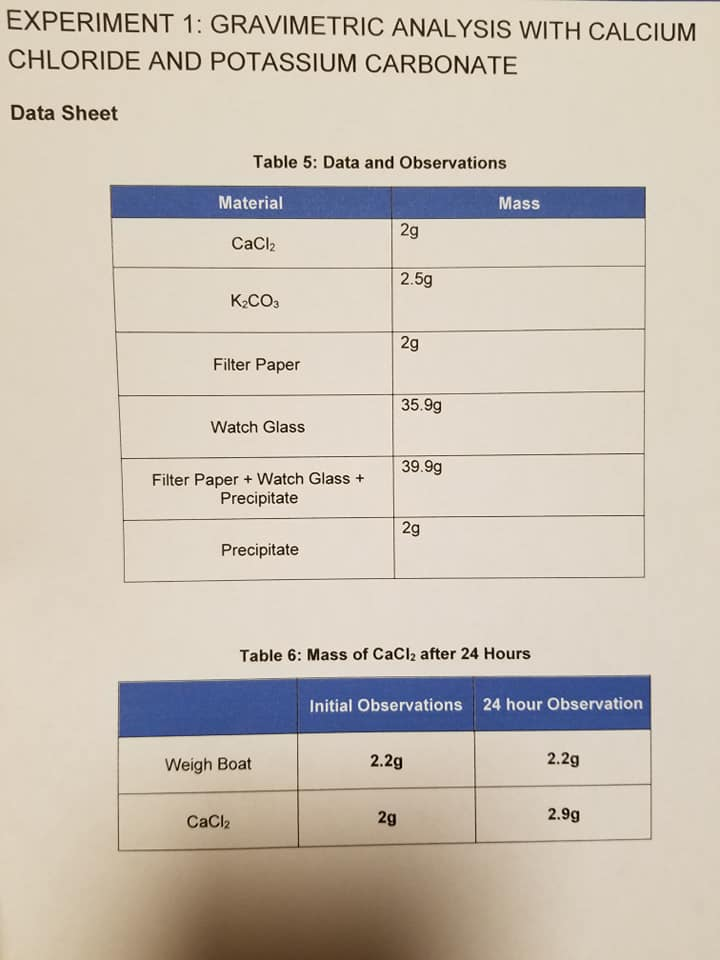
This yields 001828 moles. Grams CaCO3 mass of filter paper dried CaCO3 mass of filter paper. GRAVIMETRIC ANALYSIS WITH CALCIUM CHLORIDE AND POTASSIUM CARBONATE Data Sheet Table 5.
076 g of Ca Mass of Ca in the original solution 076 g Ca 3. All data reactions and calculations should be recorded directly into your lab notebook. Use this to find the moles of calcium carbonate.
Data and Observations Material Mass 2g CaCl2 25g K2CO3 2g Filter Paper 359g Watch Glass 399g Filter Paper Watch Glass Precipitate 2g Precipitate Table 6. Mass of filter paper 0598 g mass of filter paper dried CaCO3 2436 g. Chemistry questions and answers.
It happens that the precipitate is soluble in acidic solution because the oxalate. Dehydrating the Alkali Metal Carbonate 1. Gravimetric analysis is a laboratory technique that is used to determine the mass the mass concentration of a substance by measuring the change in the mass in this case by applying heat.
Grams anhydrous salt mass of crucible M2CO3 mass of crucible b Calculate the mass of calcium carbonate precipitated. Bicarbonate has an effervescent property that. Ca2 aq C 2O4 2-aq H 2O l CaC2O4H2O s Large easily filtered relatively pure crystals of product will be obtained if the precipitation is carried out slowly.
Analyze the data and use your yield of calcium carbonate to determine the experimental concentration of calcium chloride in the solution that you made in steps 6 and 7. Lab Report Gravimetric Analysis Of Calcium Chloride Author. Lab 16 gravimetric analysis of metal carbonate gravimetric analysis lab report 1 the writing center gravimetric analysis of an unknown carbonate a sedano gravimetric analysis of a metal carbonate by udit modi on.
The precipitated calcium carbonate is then filtered dried and weighed. Transfer unknown to weighing bottle. A solution of calcium chloride is added to the metal carbonate solution to precipitate the carbonate ions as calcium carbonate.
Calcium ion can be analyzed by precipitation with oxalate in basic solution to form CaC2O4H2O. It happens that the precipitate is soluble in acidic solution because the oxalate. The manual helps students understand the timing and situations for the various techniques.
Each experiment is presented with concise objectives a comprehensive list of techniques and. Gravimetric determination of calcium lab report cao in our unknown was found to be 44 07 introduction the point of this. Heat the crucible and carbonate form 2-3 minutes.
The Gravimetric Determination of CalciumAbstract. This experiment helps teach us the theory behind gravimetric determination as well as how to use a homogeneous precipitation to crystallize a. Explain the nature of thermogram of Calcium Oxalate Monohydrate CaC2O4H2O Lab Experiment 4.
Ca2 aq C 2O4 2-aq H 2O l CaC2O4H2O s Large easily filtered relatively pure crystals of product will be obtained if the precipitation is carried out slowly. Data and Observations Material Mass CaCl2 20 g K2CO3 25 g Filter Paper 22 g Watch Glass 349 g Filter Paper Watch Glass Precipitate 400 g Precipitate 29 g Table 2. Lab Report Gravimetric Analysis Of Calcium Chloride Keywords.
Gravimetric analysis is a method in which the application of precipitation a reaction in which an insoluble product is formed by two soluble substances reacting Kan Wu. Finally the last main goal of this lab is to calculate the percent CaO in an unknown sample1. Up to 24 cash back The purpose of this advanced inquiry lab is to investigate the suitability of gravimetric analysis for determining the amount of water hardness in the form of calcium carbonate CaCO 3 in various water samples.
A Calculate the grams of anhydrous salt in the sample. While generally a safe household remedy its high sodium content is a disadvantage. Calcium carbonate unknown Perform the analysis in duplicate.
Lab report gravimetric analysis of calcium chloride Created Date. Use this to find the moles of calcium carbonate. Record the number of your unknown in your lab notebook.
Grams anhydrous salt mass of crucible M2CO3 mass of crucible b Calculate the mass of calcium carbonate precipitated. The Beran lab manual has long been a market leading lab manual for general chemistry. Up to 24 cash back The purpose of this lab is to determine the identity of a Group 1 metal carbonate compound by gravimetric analysis.
Dividing the mass of the unknown carbonate by the moles of CaCO3 will yield the molar mass of M2CO3. Prepare the crucible by heating for approximately 1 minute allowing it to cool and finding its mass. Add approximately 2 g of the unknown carbonate to the crucible and find the combined mass of the crucible and carbonate.
The moles of CaCO3 is 001828 because the mass of CaCO3 is 1830 grams therefore in order to get the moles of CaCO3 we must divide 1830 by the molar mass of CaCO3 which is 1001. The Essay on Ap Chemistry Gravimetric Lab. Mass of CaCl2 after 24 Hours Initial Observations 24 hour.
The first main objective is to understand the theory of gravimetric analysis which will be done experimentally using CaO. Unlikely to be recommended by doctors bicarb or baking soda is still a common component of many patent medicines. Another concept in this lab is the use of homogeneous precipitation to crystallize a sample.
H2O s Soluble calcium can be quantitatively analyzed by precipitation as calcium. GRAVIMETRIC ANALYSIS WITH CALCIUM CHLORIDE AND POTASSIUM CARBONATE Data Sheet Table 1. Up to 24 cash back LAB 3 GRAVIMETRIC ANALYSIS In todays lab you will determine the identity of an unknown calcium halide CaX2 using a precipitation reaction.
Moles CaCO3 grams CaCO3 molar mass of CaCO3. This reasoning can be mathematically expressed as follows. The purpose of this experiment was to determine the calcium content of an impure sample of calcium carbonate by converting the calcium to solid calcium oxalate monohydrate.
The unknown is weighed and dissolved in water. Read Online Gravimetric Analysis Of Calcium Lab techniques. The Gravimetric Analysis of Barium Chloride Hydrate.
Mass of CaCl2 after 24 Hours Initial Observations 24. Six samples representing a wide range of potential water hardness from 50 ppm to 500 ppm. Calcium ion can be analyzed by precipitation with oxalate in basic solution to form CaC2O4H2O.
In this experiment an unknown Group 1 metal carbonate M 2 C0 3 is analyzed to determine the identity of the Group 1 metal M. With these values a molar mass of M2CO3 can be found. The precipitate is filtered dried and weighed.

Lab 16 Gravimetric Analysis Of Metal Carbonate

Solved Experiment 1 Gravimetric Analysis With Calcium Chegg Com


0 Comments